how to draw molecular orbital diagram khan academy
The HOMO and LUMO. The Aufbau principle tells you that the lowest-energy orbitals fill first but the specific order isnt sequential in a way thats easy to memorize.

Introduction To Electron Configurations Video Khan Academy
So we go back down here and we find our central atom which is our carbon.

. Lewis VSEPR Valence Orbitals and MO. Molecular Orbitals One approach to understanding the electronic structure of molecules is called Molecular Orbital Theory. This is a very basic introduction to molecular orbital theory.
See Resources for a diagram showing the filling order. It covers the basics of how to solve for bond order. There are a total of 6 electrons to add to the molecular orbital diagram 3 from boron and 1 from each hydrogen atom.
Norris discusses frontier molecular orbitals. Next were going to count the number of electron clouds surrounding our central atom. This article explains how to create molecular orbital diagrams in L a T e X by means of the package MOdiagramFor information about the more traditional molecular structure diagrams see our documentation about chemistry formulae.
Draw the MO diagram for B_2. I would also greatly appreciate a series on this topic. So your first molecular orbital should have 0 nodes and then increase with increase by one with each increasing energy level so the more energy levels you have you would just increase the number of nodes by one each.
In this case were using the standard one. So you must always be flipping it back and forth 4 the number of nodes in your molecular orbitals must always begin at 0. AO2py AO2py π2py π 2py weak sidelong overlap AO2pz AO2pz σ2pz σ 2pz strong head-on overlap Thus we take 10 atomic orbitals and generate 10 molecular orbitals in accordance with the conservation of orbitals.
Note that the n 1 level only has s orbitals the n 2 level only has s and p orbitals and the n 3 level only has s p and d orbitals. A Draw a molecular orbital MO diagram for CO and show the filling of electrons. So step 1 is done draw dot structure to show the valence electrons.
The Lewis structure shows that the beryllium in BeH 2 makes 2 bonds and has no lone pairs. Overlapping atomic orbitals produce molecular orbitals located in the middle of the diagram. Since this is just the location in which electrons can exert the most attractive force on the two nuclei simultaneously this arrangement constitutes a bonding.
In molecular orbital theory a covalent bond is formed whenever two atoms overlap all of their orbitals regardless of whether they are valence orbitals or not to create bonding and antibonding orbitals. The Y-axis of a MO diagram represents the total energy not potential nor Gibbs Energy of the orbitals. The intuition of bond order orbital conf.
Compare the bond order to that seen in the Lewis structure remember that an electron in an antibonding orbital cancels the stabilization due to bonding of an electron in a bonding orbital. Boron has 2 electrons in the 2s orbitals and 1 electron in the 2p orbital. Atomic orbitals must have the proper symmetry and energy to interact and form molecular orbitals.
It is a linear molecule. Thats it for the MO diagram of B_2. How to draw molecular orbital diagram.
In MO theory explaining bonding anti bonding and non bonding orbitals in general and how to fill the electrons in the orbitals. Molecular orbital diagram as a non-bonding molecular orbital. First step is to determine which MO diagram were using.
Photoelectron spectroscopy provides useful information on the energies of atomic orbitals. As two H nuclei move toward each other the 1s atomic orbitals of the isolated atoms gradually merge into a new molecular orbital in which the greatest electron density falls between the two nuclei. Yes this is found in p subshells when forming.
Orbital diagrams must follow 3 rules. Molecular orbitals are constructed by taking linear combinations. Sp3 Hybridized Orbitals and Sigma Bonds.
Draw out the MO diagram and label in the valence electrons. Orbital diagrams are a visual way to show where the electrons are located within an atom. Individual atomic orbitals AO are arranged on the far left and far right of the diagram.
What is the molecular geometry of BEf2. Molecular orbital diagrams provide qualitative information about the structure and stability of the electrons in a molecule. Between molecules like N2 O2 and others like HF.
Summary MO Theory LCAO-MO Theory is a simple method for predicting the approximate electronic structure of molecules. The Aufbau principle the Pau. Created by Sal KhanWatch the next lesson.
Sp Hybrid Orbitals in BeH2 1. He also discusses applications in understanding reactions and in UVvisible absorption s. General Notes on Molecular Orbital Diagrams.
Next well see that symmetry will help us treat larger. Construct a qualitative molecular orbital diagram for chlorine Cl 2. MAKE SURE TO SUBSCRIBEThis video puts emphasis on molecular orbital diagrams a fundamental way of understanding why Diels-Alder chemistry works.
MO theory assumes that the valence electrons of the atoms within a molecule become the valence electrons of the entire molecule. Label orbitals sigma sigma pi.
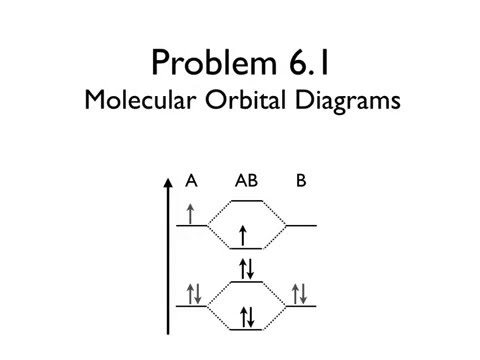
Molecular Orbital Diagrams Chemistry X Youtube
Mo Diagrams For Linear Triatomic Molecules Chemistry Libretexts

The Bohr Model And Atomic Orbitals Using An Element S Position In The Periodic Table To Predict Its P Chemistry Lessons Teaching Chemistry Chemistry Classroom

Chemistry 101 Molecular Orbital Theory Youtube
The Aufbau Principle Video Khan Academy
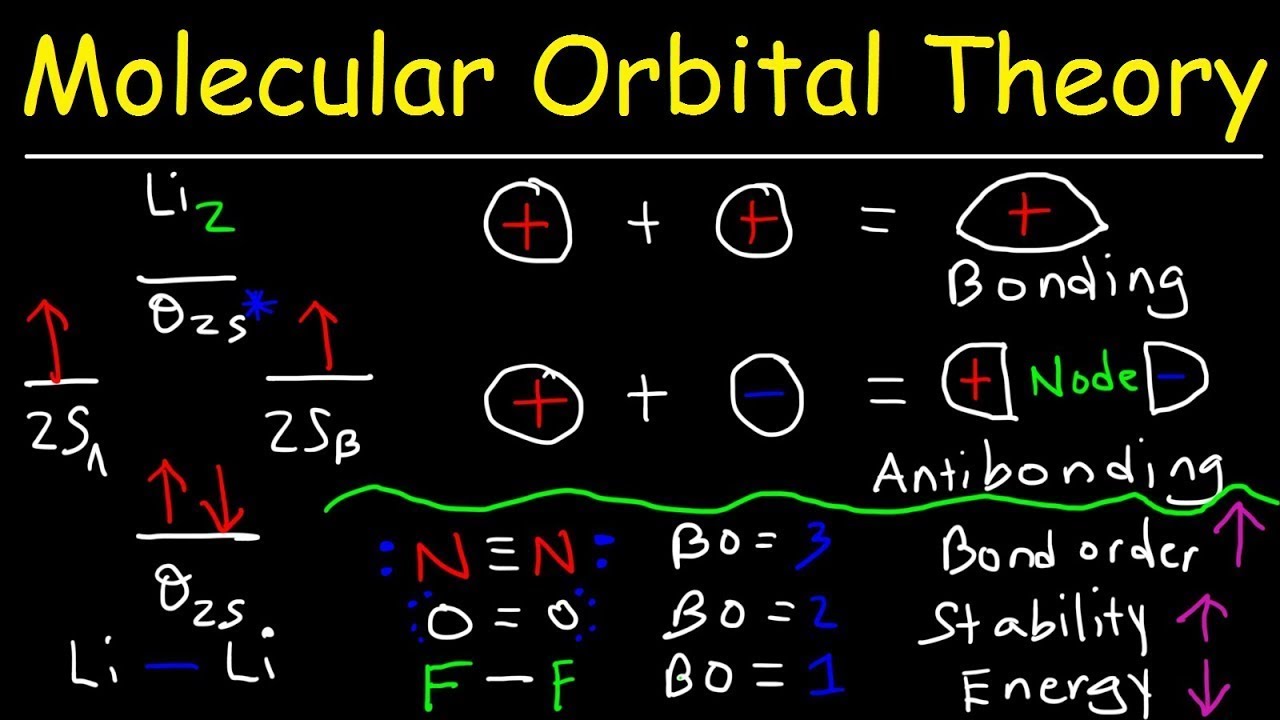
Molecular Orbital Theory Bonding Antibonding Mo Bond Order Youtube

What Is An Sp3 Hybridized Carbon Atom A Plus Topper Sp3hybridization Atom Carbon Chemistry

Molecular Orbital Theory Boundless Chemistry

Ocn Lewis Structure How To Draw The Lewis Structure For Ocn Drawings Tech Company Logos Draw